top of page



1/1

®
IMPAVIDO
Impavido® (miltefosine) is an FDA-approved treatment for cutaneous, mucosal and visceral leishmaniasis in patients 12 years of age and older.
RARE
Happens
Cutaneous
Visceral
How do people get infected with Leishmania parasites?
The main way is through the bite of infected female phlebotomine sand flies. Sand flies become infected by sucking blood from an infected animal or person. People might not realize that sand flies are present because:
-
They do not make any noise;
-
They are small: they are only about one third the size of typical mosquitoes or even smaller;
-
Their bites might not be noticed (the bites can be painless or painful).
Sand flies usually are most active in twilight, evening, and night-time hours (from dusk to dawn). Although sand flies are less active during the hottest time of the day, they may bite if they are disturbed (for example, if a person brushes up against the trunk of a tree or other site where sand flies are resting).
Some types (species) of Leishmania parasites also may be spread via contaminated needles (needle sharing) or blood transfusions. Congenital transmission (spread from a pregnant woman to her baby) has been reported.
Lifecycle in the sand fly
The sand fly feeds on blood from an infected mammal and takes up the amastigote form of the parasite into the abdominal midgut. Here the parasite transforms to the procyclic promastigote form. The parasite is then thought to attach to the midgut wall and finally migrate back towards the mouthparts. Just before it reaches the mouthparts it converts to its mammalian-infectious form, the metacyclic promastigote, ready for when the sand fly takes its next blood meal. The parasite is passed on because the sand fly regurgitates the mixture that lingers in its mouth parts into the bite before feeding.



What is Leishmaniasis?
Leishmaniasis is an important human and veterinary disease caused by Leishmania parasites that affect 12 million people in over 98 endemic countries. Leishmaniasis is a parasitic disease that is found in parts of the tropics, subtropics, and southern Europe. It is classified as a Neglected Tropical Disease (NTD). The disease is now emerging in Europe due to climate change and massive population displacement. The parasite is known to rapidly adapt to novel environments with important consequences for disease outcome. It has therefore been recognized as an emerging public health threat for the EU.
Leishmaniasis is caused by infection with Leishmania parasites, which are spread by the bite of phlebotomine sand flies. There are several different forms of leishmaniasis in people. The most common forms are:
-
cutaneous leishmaniasis, which causes skin sores,
-
mucosal leishmaniasis, which affect the nasal, oral and pharyngeal mucosa
-
and visceral leishmaniasis, which affects several internal organs (usually spleen, liver, and bone marrow).
Leishmaniasis is among the five most important parasitic diseases worldwide, with an estimated 350 million people at risk of infection. The disease causes a spectrum of clinical manifestations ranging from disfiguring cutaneous to fatal visceral forms, which results from infection by different species of Leishmania parasites. These unicellular parasites adapt to a remarkable range of hosts. They grow as extracellular parasites inside phlebotomine sand flies that transmit Leishmania to variety of vertebrates, such as rodents, dogs, and humans, where they grow inside immune cells, notably macrophages, causing severe pathologies that may lead to death.
Leishmaniasis is one of the most neglected diseases and as a consequence attracts limited attention. There is no human vaccine and only few treatments are available,.
WHERE IMPAVIDO IS ONSITE FOR AMOEBIC USE Cook Children’s Medical Center Fort Worth TX 76104 Childrens Medical Center Dallas Dallas TX 75235 Palmetto Health Richland Columbia SC 29203 James A. Haley VA Medical Center Tampa FL 33612 Carolina Medical Center Charlotte NC 28203 Orlando Health Orlando FL 32806 Johns Hopkins All Children's Hospital St. Petersburg, FL 33701 UPMC Presbyterian Shadyside Pittsburgh PA 15203 Texas Childrens Hospital Houston TX 77030 U of MN Medical Center Fairview Minneapolis MN 55454 Tampa General Hospital Tampa FL 33606 Lee Memorial Health System Fort Myers FL 33908 University Health Shreveport Shreveport LA 71103 Shands Teaching Hospital & Clinics Gainesville FL 32610 Nebraska Medicine Omaha NE 68198 Lakeland Regional Medical Center, Lakeland, FL 33805 Nicklaus Children's Hospital, Miami, FL 33155 Nemours Childrens Hospital, Orlando, FL 32827 Joe DiMaggio Childrens Hospital,Hollwood FL 33021 Arkansas Children's Hospital, Little Rock, AR 72202 Pomona Valley Hospital Medical Center, Pomona, CA 91767 Parkview Regional Medical Center, Fort Wayne, IN 46845 Vidant Health, Greenville, SC 27834 Children's Hospital Colorado, Aurora, CO 80045 UNC Health, Chapel Hill, NC 27514

In the News
What is IMPAVIDO (miltefosine)?
IMPAVIDO is a prescription medicine used to treat certain types of leishmaniasis:
-
visceral leishmaniasis (affecting your internal organs)
-
cutaneous leishmaniasis (affecting your skin)
-
mucosal leishmaniasis (affecting your nose, mouth and throat)
Impavido is the first and only FDA-approved drug to treat cutaneous or mucosal leishmaniasis, in addition to Viceral leishmaniasis.
It is not known if IMPAVIDO is safe and effective in children under 12 years of age.

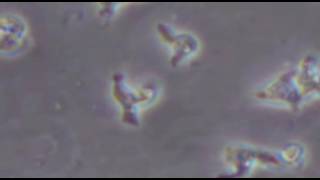
**The sand flies that transmit the parasite are only about one third the size of typical mosquitoes or even smaller. On the left, an example of a vector sand fly (Phlebotomus papatasi) is shown; its blood meal is visible in its distended transparent abdomen. On the right, Leishmaniapromastigotes from a culture are shown. The flagellated promastigote stage of the parasite is found in sand flies and in cultures. Credit: PHIL, DPDx.
IMPAVIDO Drug Description

As of 2007, at least 1300 soldiers have been diagnosed with leishmaniasis (mostly CL but a few isolated cases of VL) since deployment to both countries; many speculate that the number may be as high as 2,500, due to underreporting or misdiagnoses by physicians unfamiliar with this exotic disease
Stock Impavido In your Hospital for free* with our
consignment PROGRAM
FREE LIVING AMOEBA - Primary Amebic Meningoencephalitis (PAM) - Naegleria fowleri, Balamuthia, Acanthamoeba Keratitis
Be ready for the rare infection
IMPAVIDO capsules contain the active ingredient miltefosine, an antileishmanial agent. The chemical name of miltefosine is 2-[[(hexadecyloxy)hydroxyphosphenyl]oxy]-N,N,Ntrimethylethylammonium inner salt. Miltefosine is a white powder that is freely soluble in water, 0.1 N HCl or NaOH, methanol, and ethanol. It has the empirical formula of C21H46NO4P with a molecular weight of 407.6 and the following structural formula:


The inactive ingredients are colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
IMPORTANT DRUG SAFETY INFORMATION
Indication for Impavido® (miltefosine)
IMPAVIDO capsules contain the active ingredient miltefosine, an antileishmanial agent.
IMPAVIDO is an antileishmanial drug indicated in adults and adolescents 12 years of age
weighing 30 kg (66 lbs) for treatment of:
-
Visceral leishmaniasis due to Leishmania donovani
-
Cutaneous leishmaniasis due to Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis
-
Mucosal leishmaniasis due to Leishmania braziliensis
Limitations of use: Leishmania species evaluated in clinical trials were based on epidemiologic
data. There may be geographic variation in the response of the same Leishmania species to
IMPAVIDO (1, 14). The efficacy of IMPAVIDO in the treatment of other Leishmania species
has not been evaluated.
Important Safety Information for Impavido® (miltefosine):
IMPAVIDO may cause serious risks to pregnancy:
-
Do not take IMPAVIDO if you are pregnant. If you take IMPAVIDO during pregnancy, your baby is at risk for death or serious birth defects.
-
Women who can become pregnant should use effective birth control (contraception) during IMPAVIDO treatment and for 5 months after stopping IMPAVIDO treatment. Discuss with your healthcare provider which birth control method is right for you
-
If you become pregnant while taking IMPAVIDO, tell your healthcare provider right away. Talk to your healthcare provider about taking part in the IMPAVIDO Pregnancy Registry. This is a study to learn how IMPAVIDO affects pregnancy and babies. You can enroll in this registry by calling 1-866-588-5405
-
If you are a woman who can become pregnant and you are not willing to use effective birth control during IMPAVIDO treatment and for 5 months after treatment
Do not take IMPAVIDO if you:
-
are pregnant
-
have Sjögren-Larsson-Syndrome
-
are allergic to miltefosine or any of the ingredients in IMPAVIDO.
-
are a woman who can become pregnant and have not had a pregnancy test. Women who can get pregnant must have a urine or blood pregnancy test before taking IMPAVIDO.
Impavido® Higher Weight Patient Registry:
For patients who weigh 99 lbs or more, Impavido is administered at a dose of three (3) of the 50-mg capsules per day for 28 days.
Patients who weigh close to 99 lbs will receive more drug per pound than patients who have higher weights. For example, a patient who weighs 120 pounds will receive 1.25 mg of Impavido per pound each day [150 mg each day / 120 pounds = 1.25 mg per pound each day] whereas a patient who weighs 175 pounds will receive 0.86 mg of Impavido per pound each day [150 mg each day / 175 pounds = 0.86 mg per pound each day]. It is possible that Impavido will be less effective in higher-weight patients who receive less drug per pound than in lower-weight patients who receive more drug per pound.
If you weigh 165 pounds or more while taking Impavido (miltefosine), you are encouraged to enroll in The Higher Weight Registry by calling 1-866-588-5405
If you are exposed to Impavido (miltefosine) during pregnancy, contact your healthcare provider right away; you are also encouraged to enroll in the Pregnancy Registry by calling 1-866-588-5405
Helpful PDF Links

The most common side effects associated with Impavido® include nausea, vomiting and
diarrhea. Other side effects include abdominal pain, decreased appetite, dizziness, headache,
sleepiness, skin itching, and abnormalities in liver or kidney tests.
Tell your healthcare provider if you have any side effect that bothers you or that does not go
away. These are not all the possible side effects of IMPAVIDO. For more information, ask your
healthcare provider.
You may report side effects to FDA at 1-800-FDA-1088..
Tell your healthcare provider about all of the medicines you're taking, including prescription
and over-the-counter medications, vitamins and herbal supplements.
Tell your healthcare provider about all of your health conditions, including whether you are
pregnant, are planning to become pregnant, or are breastfeeding.
Impavido® has not been studied in children under 12.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit
www.FDA.gov/medwatch or call 1-800-FDA-1088.
Impavido® is available by prescription only. The information on this website should not take the
place of talking with your doctor or healthcare professional. If you have any questions about
your condition, or if you would like more information about Impavido®, talk to your doctor or
healthcare professional and see the full Prescribing Information.
Safety Info
Impavido is a registered trademark of Knight Therapeutics Licensed exclusively to Profounda in the USA
Impavido® (miltefosine) is an FDA-approved treatment for cutaneous, mucosal and visceral leishmaniasis in patients 12 years of age and older. PLEASE SEE FULL PRESCRIBING INFO
bottom of page